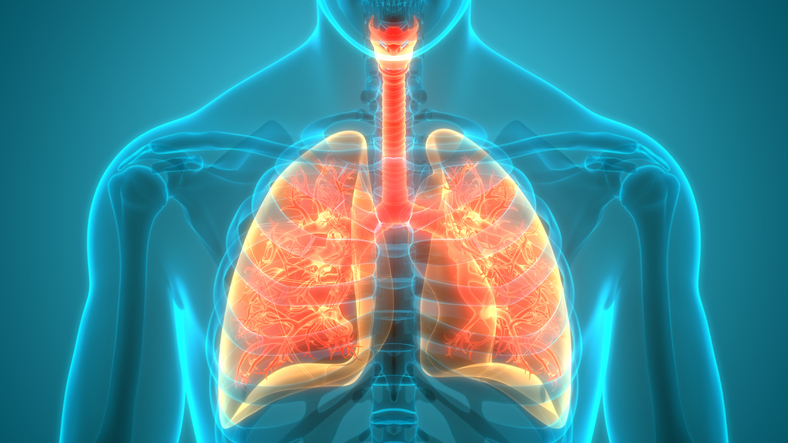
“COVID-19 is a complex disease. In some people, it can be a mild respiratory illness that is easy to recover from. In others, it can be severe and lead to a lengthy hospital stay. Patients with severe COVID-19 might need intensive care and a ventilator to help them breathe.
For people with severe illness, sometimes the only life-saving option is a lung transplant. As a pulmonologist who specializes in caring for both patients with severe COVID-19 and those who receive a transplant, I’ve seen firsthand the difference a lung transplant can make.
Here’s what our expert lung transplant team at the Temple Lung Center knows about performing these critical procedures”
Learn more here.