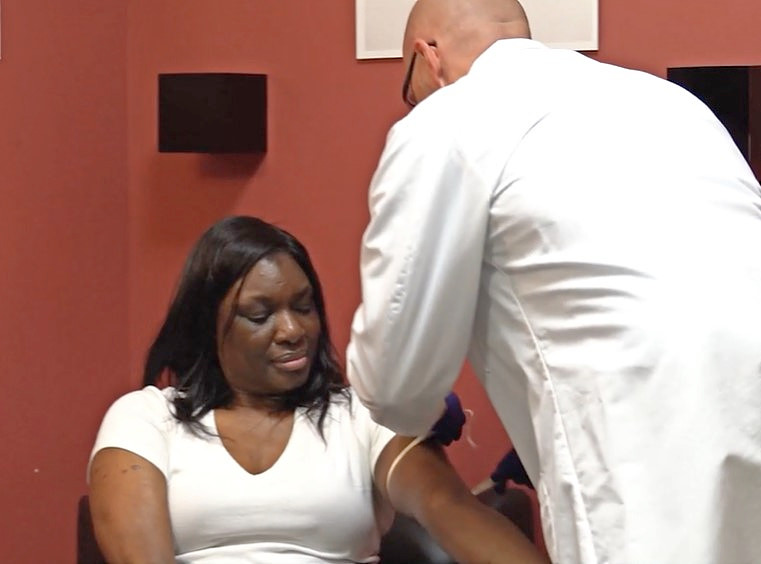
Transplant recipients never needed a COVID-19 pandemic to be anxious about infections.
Long before the pandemic, living with a transplanted organ had its own caution, stress, and anxiety about illness.
Throw social isolation into the mix and your anxiety is only made worse.
Social Distancing’s Impact on Transplant Recipients Mental Health
Isabel Stenzel Byrnes has lived 16 years with a double-lung transplant. Byrnes is a licensed social worker and grief counselor from California who says she started to feel the effects of social isolation about two months into the pandemic.
“I was starting to really find myself in a slump, and missing the energy shared with human beings,” said Byrnes, who copes by taking socially distanced walks with people.
One positive aspect of life during COVID-19 is that today we have computers, tablets, and Smartphones, which can be useful resources to visually and auditorily connect transplant recipients with their doctors, nurses, psychiatrists, social workers, colleagues, and friends.
Read the full article, here.