354310login-checkFDA OKs New Drug for CKD-Related Pruritus |
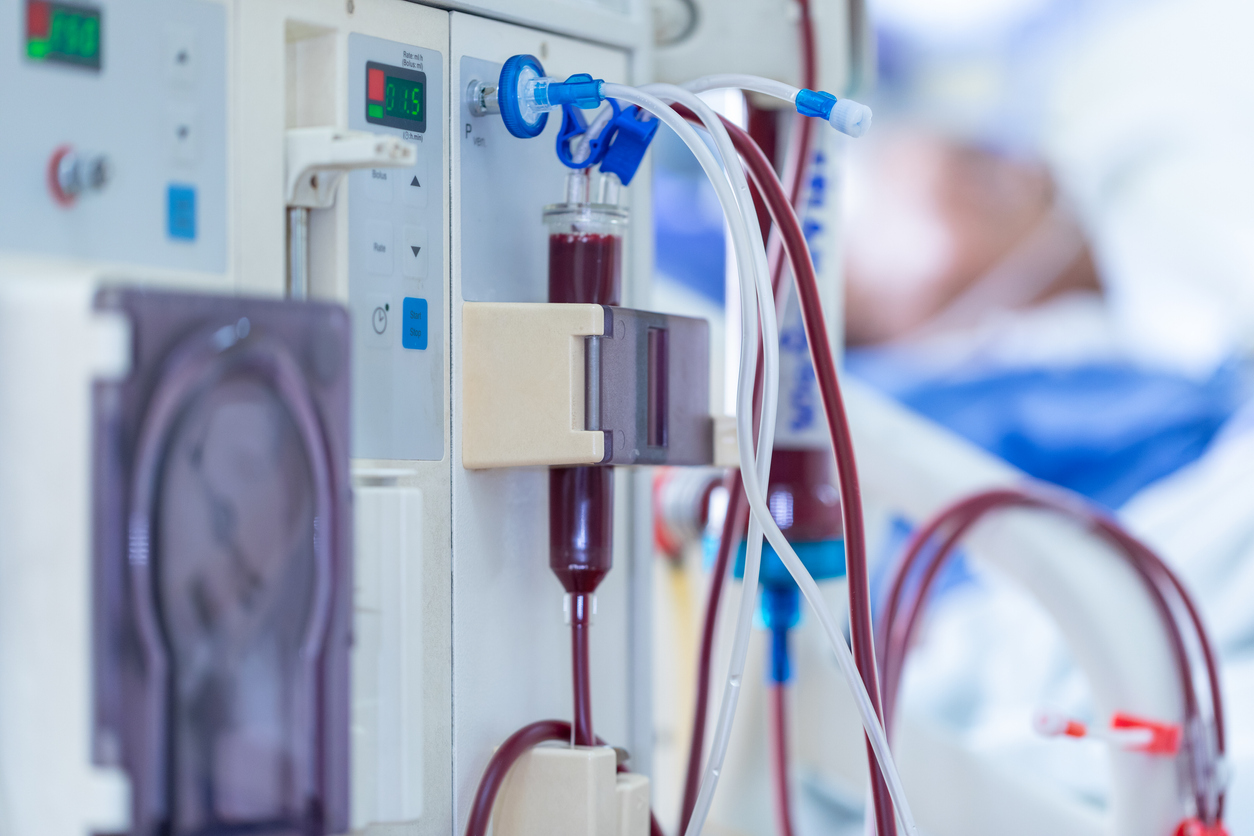
“The FDA approved difelikefalin (Korsuva) on Monday for treating pruritus in chronic kidney disease (CKD) patients, Cara Therapeutics and Vifor Pharma announced.
Difelikefalin is a first-in-class kappa opioid receptor (KOR) agonist that targets the peripheral nervous system, according to the two companies. Administered as an injection (0.5 μg/kg three times per week), the drug is indicated for moderate-to-severe pruritus in CKD patients on hemodialysis — the first therapy approved in this setting.”
Read more, here.
354310login-checkFDA OKs New Drug for CKD-Related Pruritus |